
MARERIAL PREPARATION
At Argotex, we specialize in providing comprehensive material preparation solutions to optimize your production processes. Our expert team ensures that raw materials are carefully selected, processed, and ready for seamless integration into your projects. With advanced techniques and state-of-the-art equipment, we deliver high-quality, precise, and efficient material preparation that reduce waste, improve performance, and drive superior results.
Material
Preparation

1.Solid dose

3.Powder dose

2.liquid dose & ointment & cream

4.Softgel
1.Solid dose
The preparation of solid doses, including tablets and capsules, involves a series of meticulously controlled steps to ensure high-quality, consistent products. First, the active pharmaceutical ingredients (APIs) and excipients are carefully selected and weighed according to precise formulations. These ingredients are then blended to form a homogeneous mixture.
Tablet
For tablet preparation, the mixture undergoes granulation to improve flow properties and ensure uniformity during compression. The granules are then compressed into tablets using advanced tablet presses, with precise control over key factors such as hardness, dissolution rate, and weight to guarantee consistency and quality. Our solutions also support the production of single-layer, double-layer, triple-layer, tablet-in-tablet, and effervescent tablets, enabling the development of complex formulations with controlled release, combination therapies, or specialized delivery systems tailored to meet specific therapeutic needs.
Capsule
At Argotex, we specialize in providing comprehensive material preparation solutions to optimize your production processes. Our expert team ensures that raw materials are carefully selected, processed, and ready for seamless integration into your projects. With advanced techniques and state-of-the-art equipment, we deliver high-quality, precise, and efficient material preparation that reduce waste, improve performance, and drive superior results.
COMPLETE SOLID MATERIAL PREPARATION LINE
2. liquid dose & ointment & cream
Liquid dose preparation involves the precise formulation and mixing of active pharmaceutical ingredients (APIs) and excipients to create stable and effective liquid forms such as syrups, suspensions, and solutions. The process includes dissolving or dispersing the ingredients in a suitable solvent, followed by steps like homogenization for uniformity, filtration, and sterilization to ensure safety. Rigorous quality control tests for pH, viscosity, uniformity, and stability are conducted to meet regulatory standards. Our solutions also support the development of specialized liquid formulations, including oral, topical, and injectable doses, tailored to enhance bioavailability, improve patient compliance, and meet specific therapeutic needs.
COMPLETE LIQUID MATERIAL PREPARATION LINE
3. PowDer dose
Powder dose preparation involves the transformation of liquid formulations into dry powder forms, such as those used in inhalers, sachets, or oral powders. This process can be achieved through advanced techniques like spray drying and freeze drying, each offering distinct advantages depending on the product requirements.
Spray dry
In spray drying, the liquid formulation is rapidly atomized into a fine mist and dried with hot air, resulting in a fine, free-flowing powder with a controlled particle size and moisture content. This method is ideal for creating powders with high stability, especially for sensitive active ingredients that need to retain their potency.
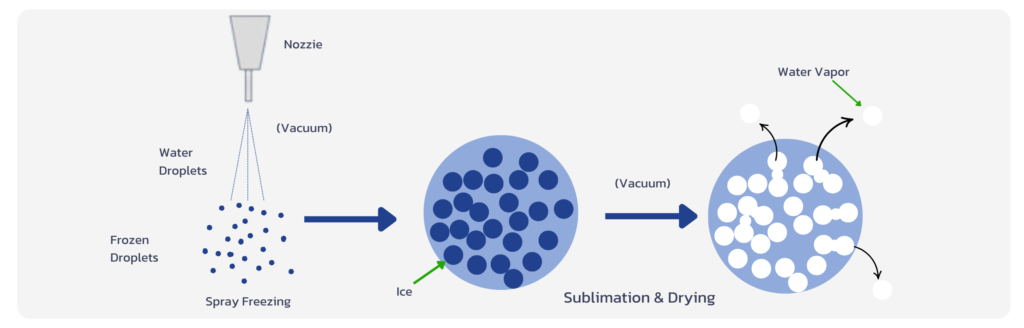
Freeze dry
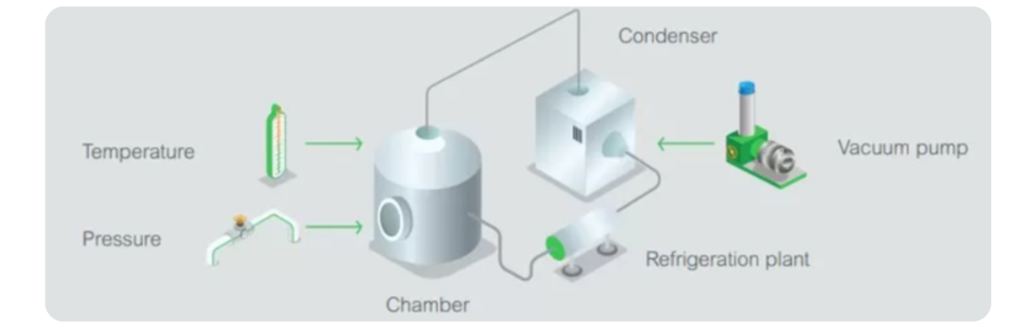
Freeze drying, or lyophilization, involves freezing the liquid formulation and then removing the water through a vacuum process. This method preserves the integrity of heat-sensitive APIs and is commonly used for biologics, vaccines, and other delicate formulations. The resulting powder is highly stable, with minimal degradation, and can be reconstituted with a solvent when needed.
4. Softgel
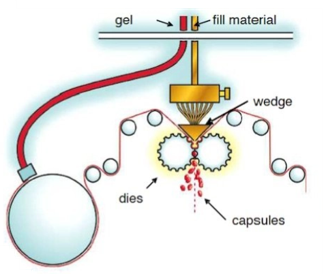
Softgel dose preparation involves encapsulating active pharmaceutical ingredients (APIs) and excipients within a soft gelatin shell to create a stable, easily administered dosage form. The process begins with the careful formulation of the filling material, which typically consists of APIs dissolved or suspended in a liquid base, such as oils or aqueous solutions. This mixture is then encapsulated within a soft, flexible gelatin shell using advanced encapsulation equipment.
THE SOFTGEL CAPSULES
The softgel capsules are produced under precise conditions to ensure
uniformity in size, fill weight, and shell integrity. The gelatin shell is typically made from a combination of gelatin, plasticizers, and water, and is designed
to dissolve quickly once ingested, allowing for fast release and absorption
of the active ingredient. Rigorous quality control measures are applied
throughout the process, including tests for fill volume, dissolution, stability
, and microbial contamination, ensuring the final product meets regulatory standards and maintains high bioavailability. Softgels are especially suitable
for formulations that require improved stability, enhanced absorption, or a
more pleasant taste, offering an effective solution for delivering sensitive
or poorly soluble ingredients.



Get a Free Consultation
from One of Our In-House Experts